Greater medical device interoperability and the adoption of commonly accepted standards could save the US in excess of $30B, suggests a West Health Institute report published in March.
Lack of device interoperability creates significant waste and risk to patient safety because of incomplete or stale information clinicians must rely on for workflow and decision making. Loosely defined standards and proprietary interfaces inhibit device “plug and play” — placing significant burden on hospitals to integrate medical devices with patient data repositories such as EHR systems.
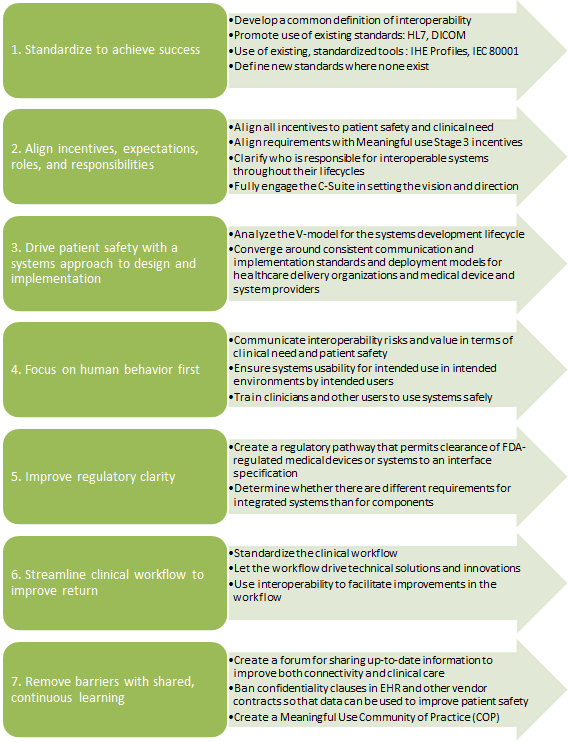
These challenges were also echoed during the AAMI-FDA Interoperability Summit in 2012 and were grouped into 7 clarion themes summarized below:
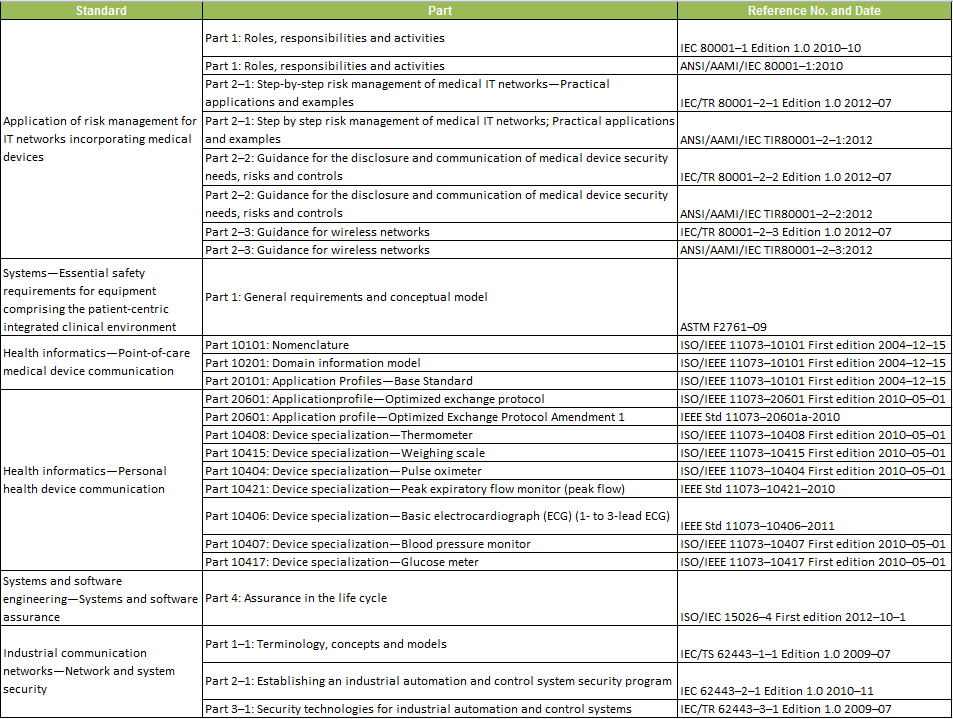
As a first step, FDA has recognized a set of voluntary standards that will help manufacturers create devices that work well together and are secure. In the Federal Register notice on August 6, FDA recognized 25 standards:
The federal government is also taking steps to broaden Stage 3 Meaningful Use requirements to incentivize greater interoperability. Other organizations like the Medical Device Plug-and-Play Interoperability Program have also been striving to advance this cause.
Still, there is a long, road ahead, and it is not an easy one. To realize the benefits, providers, and payers, medical device manufacturers and the government will need to collaborate and partner to promote device interoperability and realize its associated benefits.
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.