Jira and Confluence are popular tools for project planning and collaboration. The idea of leveraging these tools as a Quality Management System (QMS) may seem logical for businesses already utilizing them. However, despite their familiarity, Jira and Confluence are not always the best option for a QMS. While the tools have many amazing capabilities out of the box, the industry standards and regulatory compliance requirements, especially in industries like medical device manufacturing, are often too strict for the software. However, with the proper apps and add-ons, they can be transformed into a working QMS for some use cases. Let’s explore considerations for building a QMS, when Jira and Confluence can be utilized, and better alternatives.


What You Should Consider When Building Your QMS
A Quality Management System is more than a collection of tools; it’s a framework for meeting stringent regulatory and industry standards. There are a few critical factors you must consider when building one.
Regulatory Compliance
Using the medical industry as an example, a QMS must comply with:
- FDA standards (for medical device companies operating in the U.S.).
- MDR standards (for medical devices sold in the EU).
As well as globally recognized standards, such as:
- ISO 13485: Quality management system requirements for medical devices.
- ISO 14971: Risk management for medical devices.
Other common regulatory compliance standards include GDPR, FedRamp, HIPAA, and APRA.
Version Control, Auditability, and Validity
In addition to compliance, you need robust versioning capabilities to retrieve specific document versions. Furthermore, any audits you conduct must be traceable to prevent any data from going missing. Your tools must also be validated to ensure they meet the intended use in a regulated environment. This is often referred to as Computer System Validation (CSV). In many cases, this validation process must be documented.
Risk Management
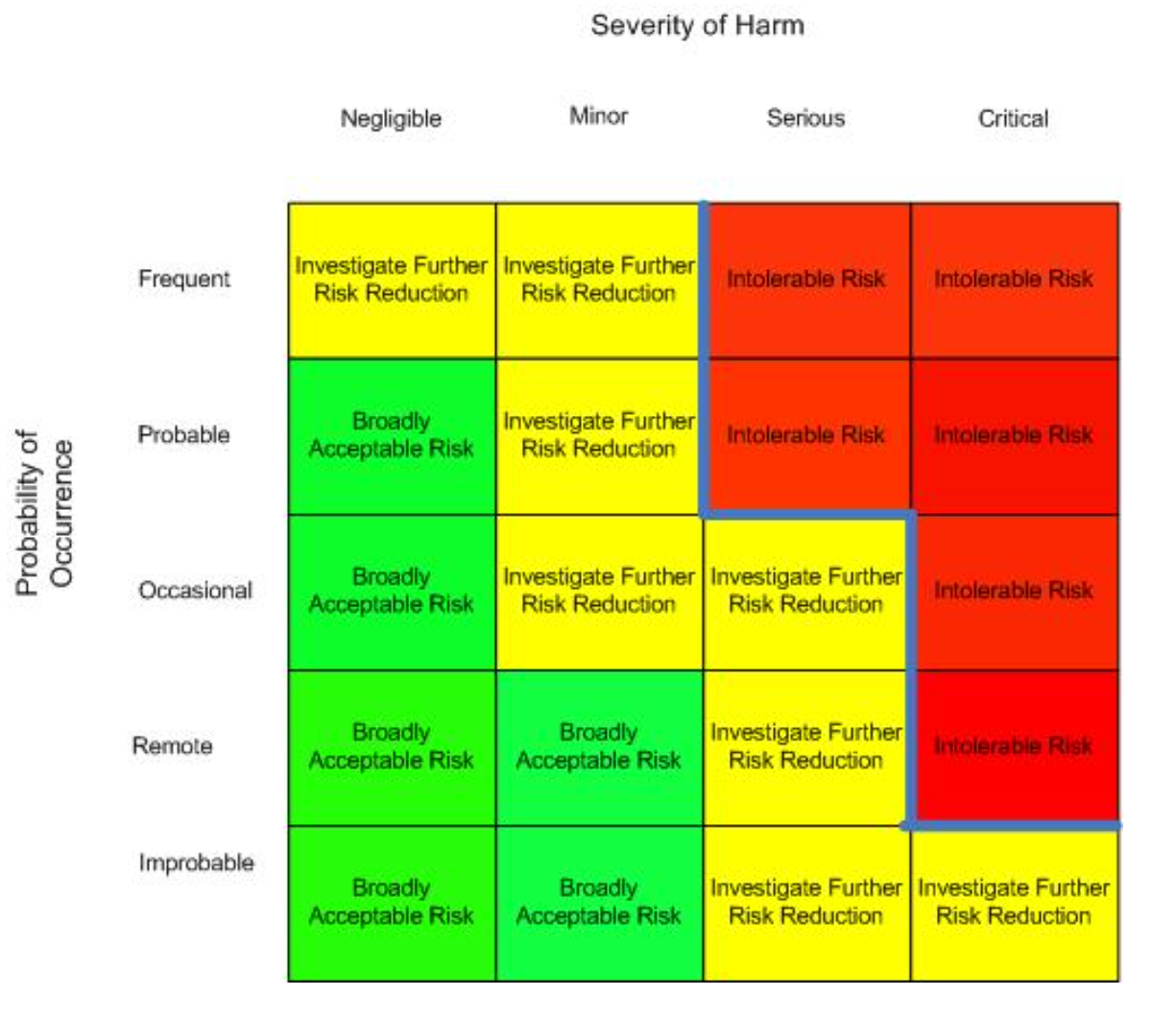
Effective risk management processes, such as Failure Modes and Effects Analysis (FMEA), are essential. Failure Modes and Effects Analysis (FMEA) is a structured, proactive approach used to analyze processes and identify potential areas where failures could occur. During an FMEA, a multidisciplinary team collaborates to predict, document, and assess the ways in which a process might fail, the causes of those failures, and their potential impact. Team members with relevant expertise then work together to develop strategies for preventing failures, with a particular focus on those that are most likely to occur or could result in significant harm to patients, staff, or the system. FMEA typically is done in a table format with calculated RPN (Risk Priority Number) seen in the figure to the right.
Scalability and Integration
The last main components of a QMS are its scalability and integration capabilities. A QMS should have the ability to scale with your organization. Furthermore, it must be able to seamlessly integrate with other tools in your environment, such as PLM or ERP systems.
Jira and Confluence as a QMS
While Jira and Confluence are excellent for general task management and collaboration, they are not designed to meet the specialized needs of a QMS without add-ons. The first reason is documentation inadequacies. For example, standards like IEC 62304 require detailed software requirements and specifications. Jira’s typical tickets or user stories fail to provide the specifications auditors need. The next reason is poor version control. Confluence’s document history isn’t able to handle versioning at a larger scale. Furthermore, manually archiving documents often causes inefficiencies.
With all this to say, there are Atlassian Marketplace Apps that can assist you in building out a QMS within Jira. For example, SoftComply offers its Risk Manager that provides advanced risk management and document control capabilities for Jira and Confluence. This is cheaper than using a stand-alone solution, and can hold up to specialized QMS platforms in certain instances. In conclusion, you can use Jira and Confluence with additional Marketplace apps as a QMS in some industries, but SPK recommends other solutions, especially for highly regulated sectors such as medical device manufacturing.
Alternatives to Jira and Confluence for QMS
If you desire a compliant, efficient QMS, consider the following purpose-built solutions.
PTC Codebeamer
Codebeamer is an all-in-one application lifecycle management tool with integrated requirements, risk, and test management. It is designed specifically for industries like medical devices, and ensures compliance with necessary ISO and FDA standards. Additionally, Codebeamer offers traceability and automated reporting to streamline audits. Lastly, it easily integrates with PLM solutions like Windchill.

Greenlight Guru
Greenlight Guru is a modern QMS designed exclusively for medical device companies. It includes features such as design controls, risk management, document management, and CAPA (Corrective and Preventive Action) processes. These features help improve speed and efficiency while reducing compliance risks.

MasterControl
MasterControl is a robust platform for document management, training, audit management, and product lifecycle management. It is trusted by regulated industries worldwide for its ability to automate compliance processes.

Ready to Implement a QMS?
Implementing a QMS can be a long, complex process, but the effort required to ensure compliance is worth it. Utilizing a purpose-built tool such as the ones we covered will help your company meet the rigorous demands of regulated industries. If you need help evaluating or implementing the right QMS for your organization, our team at SPK and Associates is here to guide you. Contact our experts to get started with a tool that provides compliance and efficiency.