When it comes to IT solutions, most mid-market manufacturing companies rely on one of two options. They have a dedicated in-house IT team, or an outsourced IT managed services provider (MSP). But manufacturing companies who care about product development and...
Data Engineering
Five Reasons to Use SolidWorks PDM in the Cloud
Tired of dealing with in-house PDM issues, unreliable VPN connections, and struggling with remote file access? Perhaps you're looking for a solution that addresses your company's specific needs. Look no further: SPK and Associates SolidWorks PDM in the Cloud presents...
Efficient Reporting in Windchill RV&S (PTC Integrity)
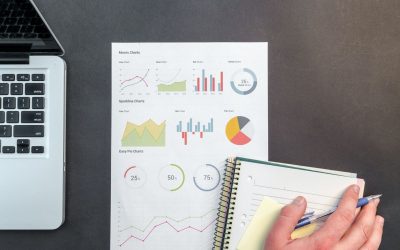
As explored in one of our previous articles, tracking metrics is a crucial component of a successful development system. But how can you leverage those metrics further? The solution is quite simple: with the trace reporting function available in Windchill...
How to Increase Uptime of Your Engineering Systems
Picture this: all your engineering applications run slow, or worse, continue to shut down. Yet, the IT team reports no alerts within the remote monitoring and management systems despite the problem. What is the root cause and how do you fix it? Remote Monitoring and...
How to Create Easy Engineering Design Automation Fixes
In one of our recent articles, we highlighted opportunities for automation, specifically for MCAD (mechanical computer-aided design) and PDM (product data management) activities in your organization. In this next installment, we hope to provide you with other examples...
How to Maximize Engineering Design Automation for CAD/PDM
In recent years, interest in engineering design automation across multiple industries skyrocketed, especially due to the high return on investment (ROI). Relieving your engineers from tedious, error-prone tasks and directing them toward automation presents a fantastic...
How Workflow Integration Boosts Engineering Productivity
How to solve the problem that hides in plain sight Engineers rarely talk about “workflow integration” for items like CAD files or project data management (PDM). They’re too busy doing their jobs! However, that exact mindset presents a major issue. Many engineers think...
Improve your build/test/deployment with CloudBees CD (formerly Electric Cloud Flow)
Repeatability is an intuitive aspect of daily life. If you throw a ball up, it comes down. If you throw it up twenty times, the same thing will happen, up and then down twenty times. When repeatability doesn’t occur and it’s supposed to, we get anxious. ...
What Is A Digital Twin And Why Is It Important?
Manufacturing companies, even the ones that are not highly regulated, have no shortage of complexity these days as they build, scale and manage multiple interdependent pieces of equipment, software bundles and networks. All this, while under time and cost pressures....
Streamline Business Communication with Google Forms
Have you ever sent out an email with a bunch of important questions and received a reply that began with "my answers are in blue". Have you then found yourself combing through your large email searching for those answers only to discover that your email provider...
PTC Integrity – Risk Management Demo (out of the box)
Cras suscipit orci at augue volutpat, ut luctus sapien ultricies. Nam vitae dolor sit amet quam pharetra scelerisque. Donec efficitur lacus felis, at ullamcorper enim molestie id. Aliquam faucibus semper neque et scelerisque. In ultrices iaculis sem metus.
Can IoT Jump These Five Hurdles?
Aliquam erat volutpat. Donec vel neque a augue interdum eleifend. Suspendisse potenti. Nulla facilisi. Integer luctus massa ut ligula tincidunt accumsan. Proin a ullamcorper sem. Etiam at erat quis justo sollicitudin hendrerit. Aliquam massa ligula nullam.