Compliance with regulatory standards is the number one priority for every medical manufacturer. Ensuring pipelines are secure and compliant doesn’t just ensure safety, but it results in better quality products. When a startup medical manufacturing company contacted...
Medical Device Engineering
How Medical Device Engineers Can Streamline CAD Workflows for Faster FDA Approval
Balancing speed and compliance in medical device engineering can be challenging. Engineers face tight deadlines and detailed requirements to bring life-saving products to market. Your CAD workflows should aid in the path to compliance and accelerate FDA approval. As...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
The FDA UDI Rule: 5 Things You Need to Know
The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know: 1. What is the UDI Rule? In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with...
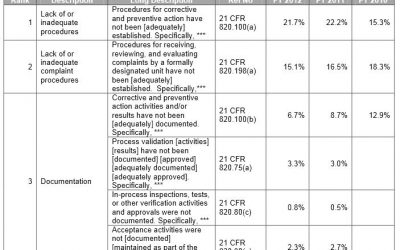
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
Accelerating Medical Software Compliance and Efficiency with SPK ACEs
Compliance with regulatory standards is the number one priority for every medical manufacturer. Ensuring pipelines are secure and compliant doesn’t just ensure safety, but it results in better quality products. When a startup medical manufacturing company contacted...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...
A Breakthrough in Custom Medical Implants with Creo
New medical approaches combined with innovative technologies are transforming how medical professionals treat patients. In this case study, we will explore how a young patient’s life was changed through the use of PTC Creo’s extensive capabilities. “Bioactive printed...
Strategic Partners, Not Just Extra Hands
Today’s business environment requires companies to align with best-in-class vendors to be true, strategic partners. They need partners who understand their industry, their challenges, and their long-term goals. In the years we have partnered with our clients, some...
Navigating Compliance and Cyber Security Concerns in Smart Medical Devices
A COMPLETE OVERVIEW OF SMART MEDICAL DEVICES, THEIR DEVELOPMENT AND THEIR COMPLIANCE CONSIDERATIONS. While the Internet of Things offers great opportunities both for businesses and consumers, compliance and security must be thought through carefully from the very...
Agile Software Development and Jira Software
Unlocking the Power of Collaboration and Efficiency Tired of trying to overcome the pain points of traditional software development methods? Frustrated with slow project delivery? Struggling with team collaboration? Version control nightmares? Worry no more. You can...
Product Lifecycle Management (PLM) Automated Metrics
Our client is a leading medtech company with 46k employees. Earlier this year, they faced the challenge of improving the efficiency of their Product Lifecycle Management (PLM) process. To stay competitive, they needed to identify bottlenecks within their...