Regulatory compliance is not just a checkbox, but a critical element for building customer trust. Despite its importance, managing compliance is not without its challenges. Complex and evolving standards can require significant coordination across teams and technologies. Regulations often require meticulous record-keeping to meet audit and control requirements. However, for companies leveraging Atlassian Cloud, the days of manual record-keeping may be over. In recent years, Atlassian has made major strides in enhancing its cloud offering to include compliance-focused features. These advancements help address common compliance challenges across industries, making Atlassian Cloud a powerful tool for organizations aiming to meet regulatory requirements. Below, we explore how Atlassian Cloud’s compliance features and add-ons can support your company’s regulatory journey.

Atlassian Cloud’s Commitment to Compliance
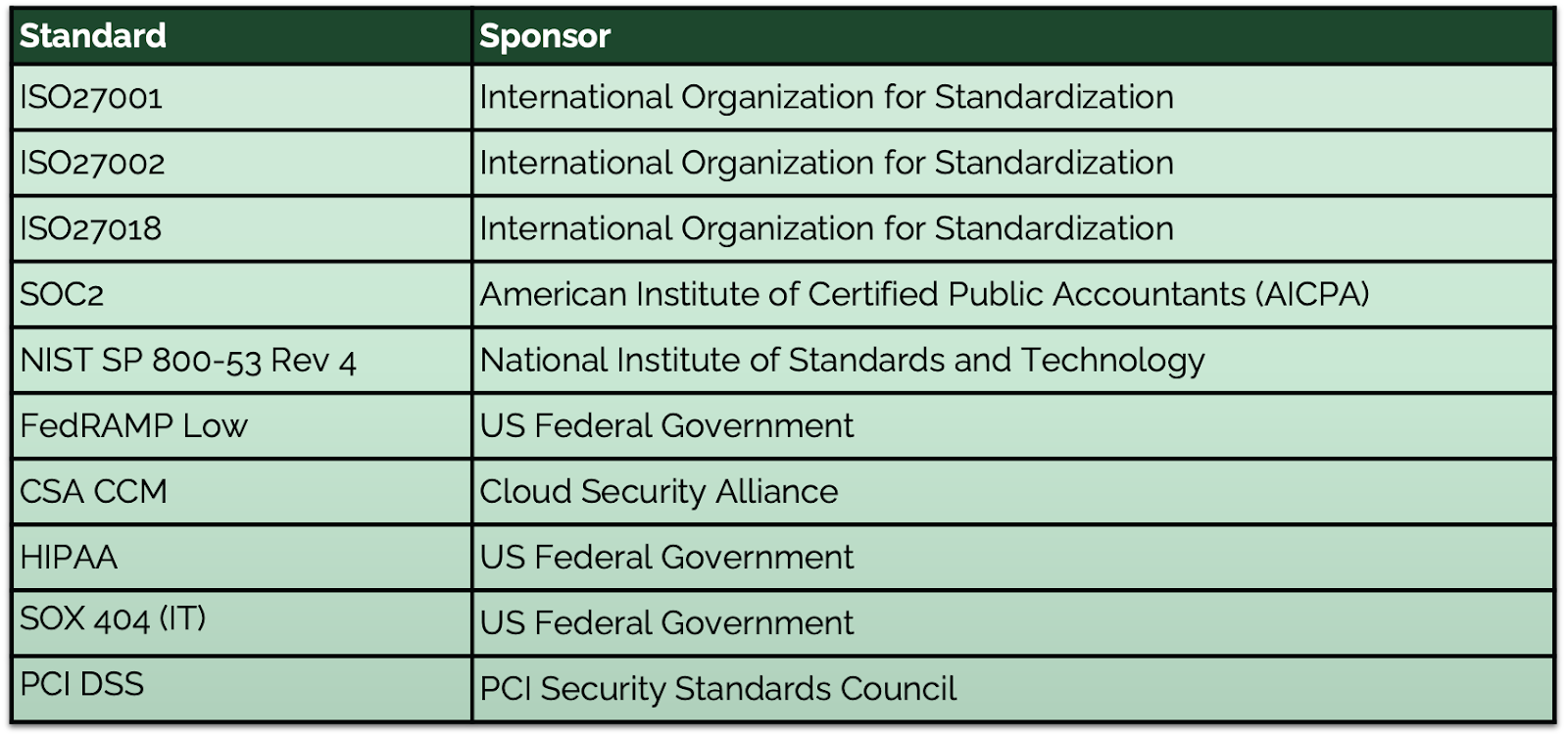
Atlassian Cloud has achieved over 28 global certifications, demonstrating compliance with industry standards like ISO, GDPR, BaFin, and HIPAA to name a few. These certifications make the offering a great option for organizations in highly regulated industries that need compliance solutions built into its architecture. Currently, Atlassian Cloud offers compliance standards for almost all industries, with one major exception: FedRAMP Moderate certification. While Atlassian has stated its FedRAMP certification is in motion for 2025, current companies needing FedRAMP Moderate compliance can partner with SPK and Associates. Our experts can host Data Center versions in GovCloud environments, ensuring your systems meet government standards.
Essential Atlassian Tools for Managing Compliance in Highly Regulated Industries
Organizations in sectors like healthcare, medical devices, and aerospace face strict regulatory frameworks to keep clients safe. Here’s how Atlassian Cloud, along with some key marketplace add-ons, can support compliance across these industries:
FDA Part 11 Compliance with Jira Cloud
FDA 21 CFR Part 11 compliance is essential for digital health and medical device companies operating in the U.S. Part 11 mandates stringent requirements for electronic records and signatures, requiring companies to demonstrate that documentation is accurate, secure, and authenticated. In Jira Cloud, companies can configure issue records to meet Part 11 requirements. By integrating apps such as eSign for Jira or SoftComply eQMS, companies can automate documentation, track changes, and ensure audit readiness. This approach covers elements like:
- The full history of issues or audit trails
- Custom fields, attachments, and user signatures
- Export-ready data with timestamped export details for FDA inspections
This setup provides companies with a streamlined way to meet Part 11 standards while improving transparency and traceability within Jira.
Streamlining GDPR Compliance with Jira Service Management
GDPR compliance is a European standard regarding the handling of personal customer data. Jira Service Management can be configured to manage personal data while remaining in compliance with this regulation. Apps like the GDPR Compliance Assistant for Jira from the Atlassian Marketplace automate personal data handling, helping to ensure GDPR-compliant workflows and reduce risk. This tool also offers data residency options, allowing companies to control where sensitive data is stored. This is crucial for organizations in finance, government, and healthcare.
Managing ISO 9001 and ISO 13485 Compliance in Jira Cloud
Companies seeking ISO compliance in manufacturing and medical devices may need additional features in Jira Cloud, such as versioning and change history. Apps from the marketplace, like SoftComply Validation, support these capabilities with audit trails, version controls, and permission management that meet ISO standards. This setup simplifies compliance management, making it easier for companies to oversee the complete lifecycle of documents and maintain a compliant QMS.
Compliance Best Practices for Atlassian Administrators
To prepare for compliance audits and ensure regulatory standards are continuously met, Atlassian administrators and IT teams should focus on the following practices:
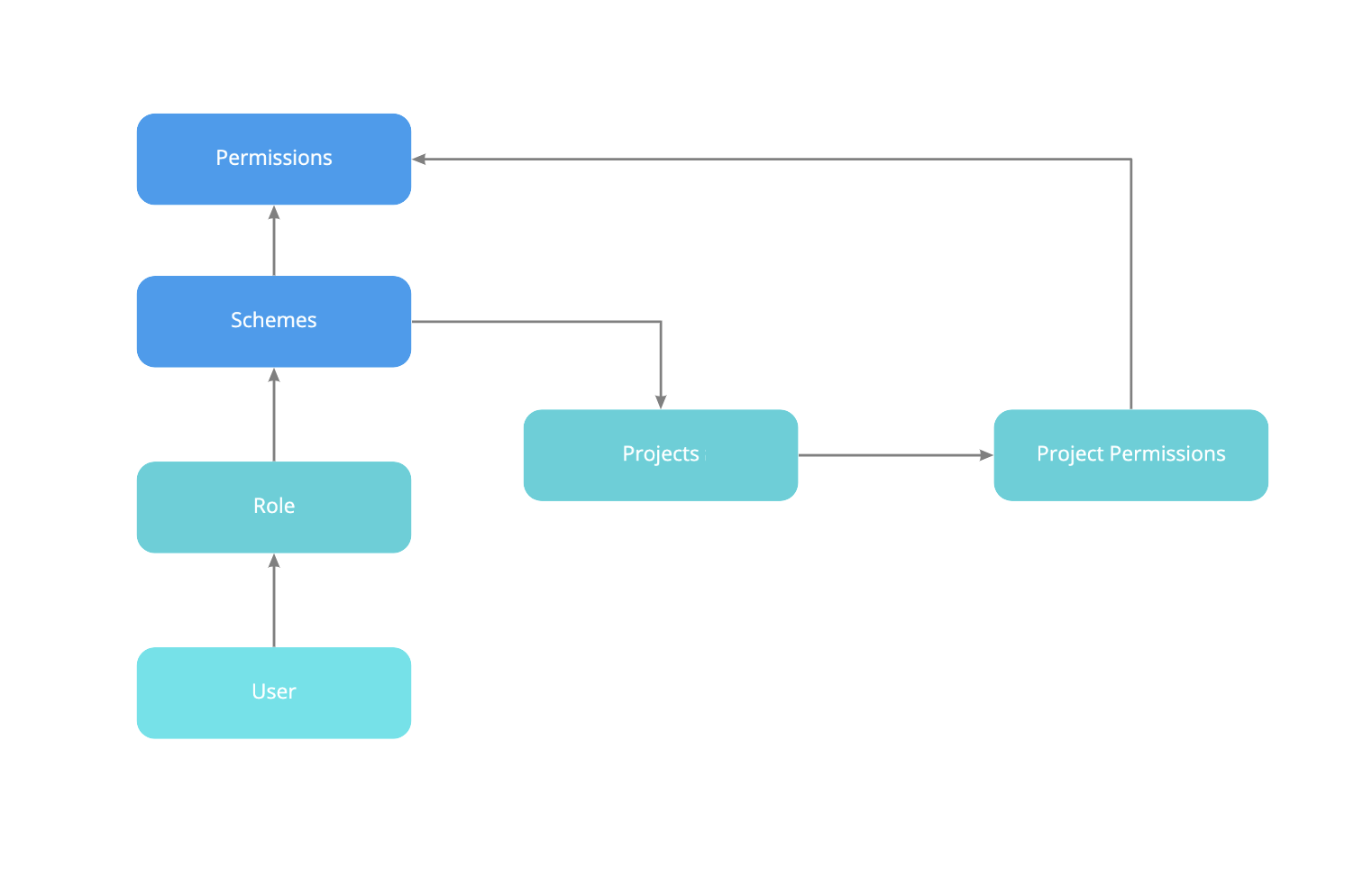
- Access Control and Permissions
Review user roles and permissions in Jira, JSM, and Confluence to ensure only authorized personnel can access sensitive data. This helps prevent unauthorized access, reducing compliance risks.
2. Comprehensive Documentation
Maintain clear documentation of system configurations, including workflow changes and plugin installations. This documentation provides auditors with context, ensuring transparency and accountability in system setups.
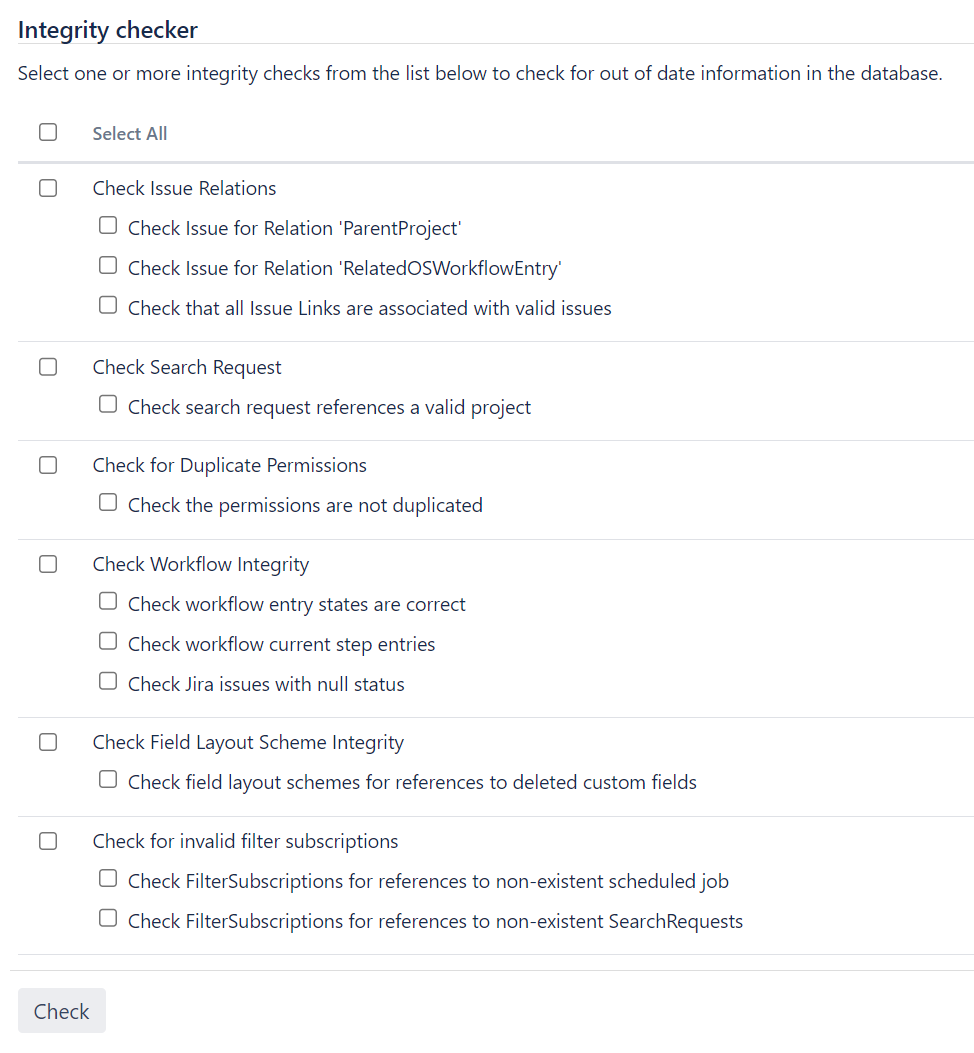
3. Data Integrity Checks
Regularly check data integrity in Jira, JSM, and Confluence, ensuring that records are complete, accurate, and consistent. This aligns tracking and service data across tools, maintaining compliance with data accuracy requirements.
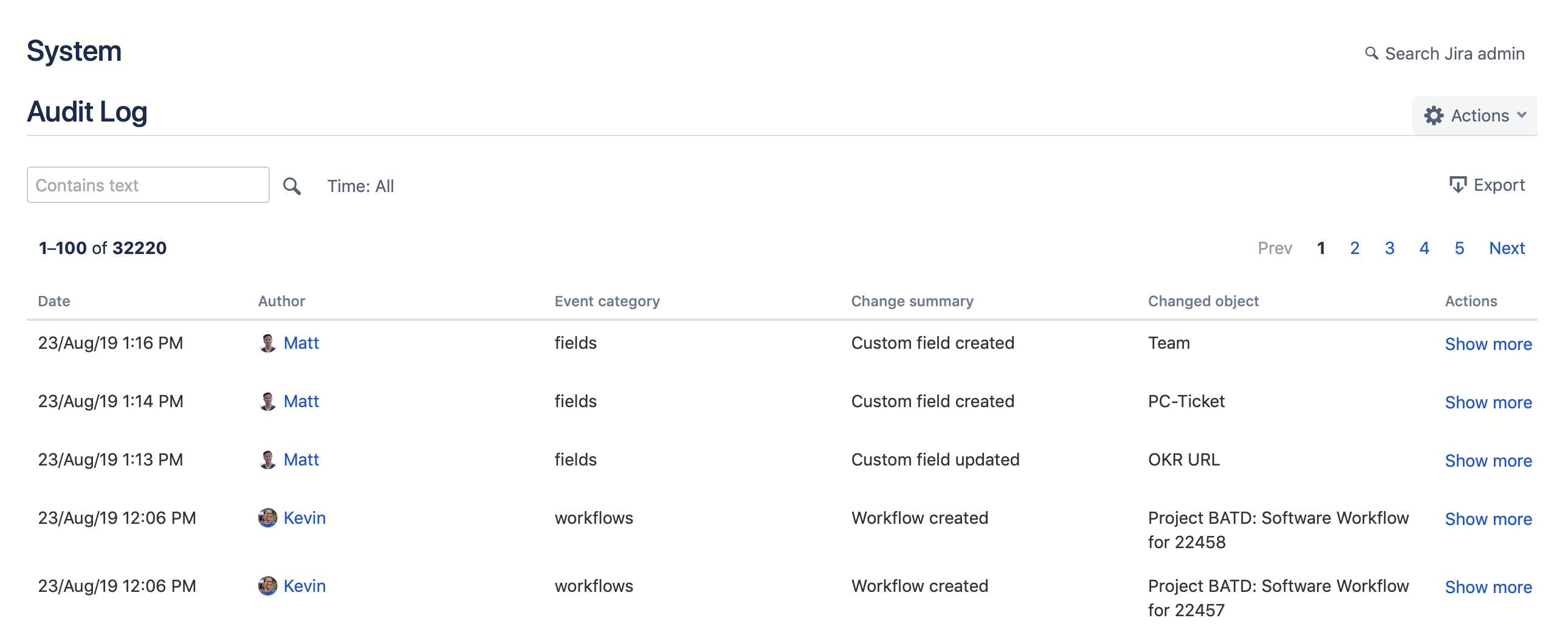
4. Audit Logs and Data Extraction
Enable audit logs to track system changes and user actions. These logs are vital to audit trails, making it easy to demonstrate control adherence. Additionally, establish procedures for data extraction, enabling quick access to project reports, issue histories, and user activities.
5. Data Backups and Recovery
It’s a common misconception that cloud storage ensures complete data security. In Atlassian Cloud, data recovery is often limited to data center-level incidents. Apps like Revyz for Jira and Confluence provide regular backups, allowing companies to restore data as needed, ensuring readiness for any compliance scenario.
Marketplace Apps for Enhanced Compliance Capabilities
In addition to the compliance configurations in Atlassian Cloud products, here are a few marketplace tools that can further strengthen compliance efforts:
- Revyz for data backup and recovery
- SoftComply Risk Manager for ISO and FDA compliance
- Stiltsoft’s izi apps for tracking employee training and certification
These tools are particularly useful for organizations needing additional control over records, audit trails, and training verification, supporting a more thorough approach to regulatory compliance.
Managing Compliance Requirements with Atlassian Cloud
Maintaining compliance can feel overwhelming, but Atlassian Cloud, combined with its compliance-driven architecture and marketplace solutions, empowers companies to meet industry standards. From secure data management to customizable workflows, Atlassian Cloud addresses key regulatory requirements across sectors, including healthcare, finance, and manufacturing. By taking advantage of Atlassian’s compliance features and recommended add-ons, your organization can simplify compliance processes, keep data secure, and stay prepared for audits. Reach out to our team to learn more about configuring your Atlassian Cloud environment or explore how SPK and Associates can support your compliance journey with expert guidance and industry knowledge.