Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
Medical Device Engineering
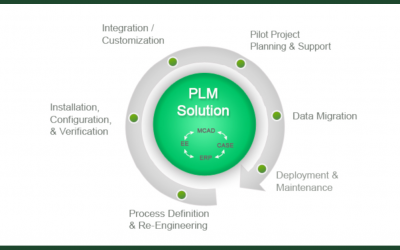
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
Accelerating Product Development with Engineering Operations
What is Engineering Operations (EngOps)? Why do you need it, and how do you effectively deploy it?There’s a strange interplay between IT and engineering teams in most firms. That’s because the tech stack that engineers need to design, build, test, and release products...
Difficulty of BOMs Across Engineering Disciplines (Part 1)
SPK and Associates co-founder Chris McHale speaks with PLM expert Oleg Shilovitsky, founder of BeyondPLM.com, to get his insight on the difficulty of BOMs across engineering disciplines.
The State of Digital Quality Maturity in Pharma and Medtech
Organizations in the medical, pharmaceutical, and life science industries are constantly adapting to their field’s rapidly changing technology and regulations. This continuous adjustment can become exhausting. Between this burnout and being unsure if your technology...
Introduction to ISO 14971:2019 for Medical Device Compliance
Navigating the complexities of ISO 14971:2019 can be challenging. But, with this eBook presented by SPK and Associates and PTC, you're taking the first step towards expertise in medical device compliance. What's In The ISO 14971:2019 eBook This comprehensive guide...
2022 Guide: Software as a Medical Device (SaMD)
What is software as a medical device? Software as a medical device, or SaMD is software that is intended for one or more medical purposes. This software performs those purposes without being part of a hardware medical device. SaMD devices also need to meet the...
Insights from Greenlight Guru’s 2023 MedTech Industry Benchmark Report
In a year of unexpected twists, the medical technology (MedTech) industry finds itself amidst shifting sands. So, Greenlight Guru’s 2023 MedTech Industry Benchmark Report is a treasure trove of insights into MedTech trends and the landscape. Let's deep dive into some...
Using Creo to Meet Stringent Regulatory Requirements in Medical Device Engineering
All medical device manufacturers understand that precision, safety, and regulatory compliance are vital in their industry. Without these precautions, individuals could be harmed. These strict requirements and extended life cycles often make the time between designing...
Blog: Leveraging PTC’s Integrity Platform for IEC 62304 Compliance
SPK and Associates leverage PTC’s Integrity platform to help Medical Device companies develop software efficiently while achieving IEC 62304 compliance.
Application Management Reduced Testing Time from Days to Under One Hour
When a global leader in infusion therapy, pain management technology, and support was developing more complex systems, they required an effective design control solution to support those efforts. SPK stepped in to help the medical company implement Windchill...
Optimizing Solidworks PDM Implementation
When multiple employees of a Fortune 100 medical equipment manufacturing company reached out to us for support with workflow functions and permissions, we knew there was a better way. Rather than fixing the issues that would inevitably keep occurring with their...