An important part of creating any product intended for use in any regulated industry vertical is that the manufacturer should have identified all of the risks involved in using the device. Then, they must have done their best to mitigate those risks before their product ever goes out to the marketplace. With this in mind, PTC bundled a Risk Management module as part of some of their off-the-shelf solutions to be used with Windchill Requirements, Validation & Source. For this article I am going to dive deeper into Risk Management.
Risk Management, as defined in ISO 14971, among other things covers the following: Risk Analysis, Risk Evaluation, and Risk Control.
Risk Analysis
Risk analysis consists of the following three steps:
- Determine the intended use and the characteristics related to the safety of the medical device.
- Identify the hazards of the medical device. These are an abstract view of what the risks may be for the patient.
- Estimate the potential outcome for each of the identified hazards.

Risk Evaluation
Once you have analyzed the risks associated with your new product, you need to perform an evaluation of the risks. Risk Evaluation consist of the following three items:
- Identify the potential sources of the hazards you identified in the phase above. Each of these potential sources would be considered a Risk.
- Determine the severity of each Risk as well as the probability of occurrence. This means you need to determine what could happen to the patient, as well as how likely it is for that outcome to occur.
- Based on the severity and the probability of occurrence, you can then determine if the Risk is “Acceptable,” “Unacceptable,” or “Needs Investigation.”
Any risks that are either Unacceptable risks, or risks that require further investigation, need Risk Control.
Risk Control
The risks can be controlled in the following manner:
- You first perform a risk control option analysis. An unacceptable or needs investigation risk can be controlled in any of the following three ways:
- Inherent safety by design. This is where you change the design of your product to reduce or eliminate the risk.
- A protective measure. This is where you add a safety feature to the product to reduce the risk.
- Provide information for the safety of the user. This is where you provide information about the specific risk, and how the customer should safely use the product.
- Once risk controls have been implemented, a residual risk evaluation is needed. The new reduced risks will still require risk control, and possibly new risks were introduced as a result of the risk control measures implemented.
- Document risks arising from risk controls. Document the overall completeness of risk control.
Of course, once you have completed those three parts (Risk Analysis, Risk Evaluation and Risk Control), you need to evaluate overall residual risk acceptability before you can move forward with the product. All of this would be bound together in a Risk Management Report that can be signed off by the appropriate stakeholders.
Risk Management in Windchill RV&S
PTC, in order to implement this in their given solution for Windchill Requirements, Validation and Source, chose to create three new document domains called Hazard, Risk, and Risk Control Measure to handle the Risk Analysis, Risk Evaluation, and Risk Control steps respectively. The diagram below explains how each of the three new domains are traced to each other and how they can connect into the Requirements Management portion of the given solution.
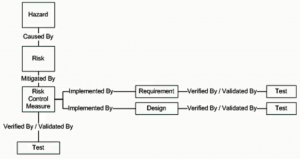
As you can see, Hazards in the Hazard document are the result of the Risk Analysis step. These Hazards are traced via the “Caused By” trace relationship to Risk document. You perform your evaluation of each risk within the Risk document. Those risks that require mitigation are traced by the “Mitigated By” trace relationship to the Risk Control Measure document. These control measures are integrated into Requirement Specification, Design Specification, and Test Protocol via the “Implemented By” and “Verified/Validated By” trace relationships.
The PTC out-of-the-box risk management solution is a good starting point for most companies. SPK and Associates focuses on tailoring the Windchill RV&S architecture to meet your specific business needs. We’ve done this for several large and small medical device manufacturers. Call us today to implement/transform your risk management process leveraging Windchill RV&S.
Next Steps
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.
- Subscribe to our blog to stay informed on product development and engineering efficiency topics.