As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant.
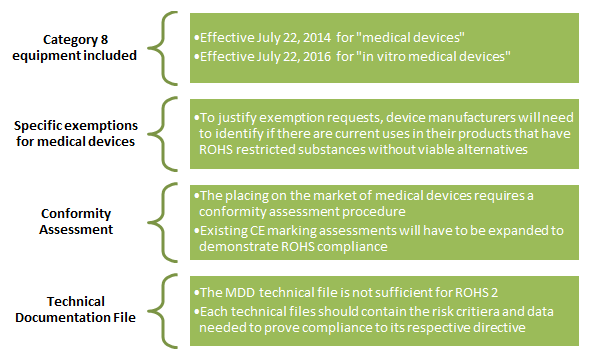
ROHS 2 Compliance Changes at a Glance
While no new substances have been added to the restricted substances, there are major changes to compliance requirements:
Practical Implications for Medical Device Manufacturers
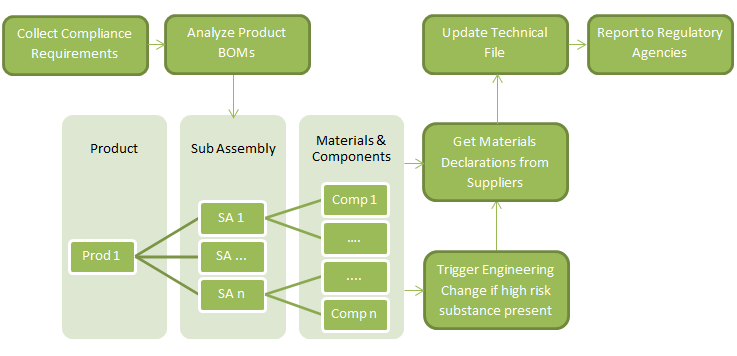
Complying with ROHS 2, in practice, will require the following steps:
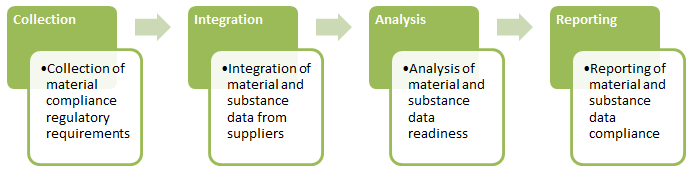
How PLM Solutions Help Design for Environmental Compliance
As illustrated above, designing for environmental compliance requires four critical phases:
Comprehensive product lifecycle management (PLM) frameworks automate these phases along the entire product lifecycle. By integrating compliance data with their PLM systems, companies can view regulatory requirements, engineering bill-of-materials (EBOMs), manufacturing BOM (MBOM), and part material and substance data related to all product information, all in a single system, allowing for a rapid response to regulatory and customer demands.
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.