Executive Summary That’s the year public and private cloud-based solutions are set to surpass traditional data centers in terms of overall spending. In 2017, nearly two thirds of spending went toward private cloud solutions. Cloud usage exploded in 2017, to the tune...
Compliance & Regulatory
How AWS Cloud Solutions Can Help Your Manufacturing Enterprise Maintain Its Competitive Edge
Executive Summary In 2020, public and private cloud-based solutions like Amazon Web Services surpassed traditional data centers in terms of overall spending. In 2017, nearly two thirds of spending went toward private cloud solutions. Cloud usage exploded in 2017, to...
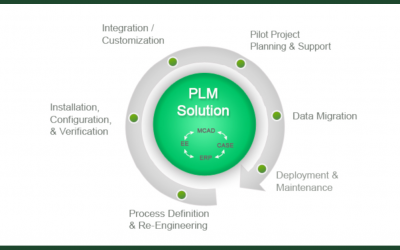
PLM: Automate your Product Development Compliance Process
Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
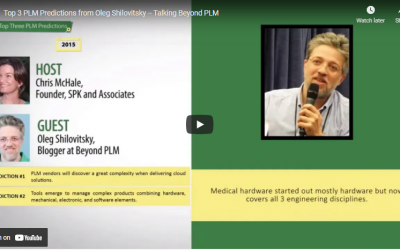
Top 3 PLM Predictions from Oleg Shilovitsky — Talking Beyond PLM
SPK and Associates co-founder Chris McHale spoke with PLM expert Oleg Shilovitsky, founder of BeyondPLM.com, to get his top three product lifecycle management (PLM) predictions for 2015. PLM vendors will encounter greater complexity when delivering cloud solutions New...
Challenges of Managing a BOM Across the Product Life Cycle (Part 2)
SPK and Associates co-founder Chris McHale spoke with PLM expert Oleg Shilovitsky of BeyondPLM, on the difficulty of BOMs across engineering disciplines. In part two of the discussion, they focus in on the difficulty of BOMs across the product life cycle.
SOTIF ISO/ PAS 21448 Guide eBook
As autonomous technology outpaces regulatory standards, further focus is needed to ensure the safety of self-driving vehicles. SPK and Associates, in collaboration with PTC SOTIF ISO/PAS 21448, brings you an insightful guide - a comprehensive eBook on the Safety of...
Excellence in Automotive Software Engineering eBook
ALM tooling, when implemented correctly, can support automotive software engineering, Agile transformation journeys and linking requirements to business strategy, encouraging collaboration. Additionally, it can be helpful for providing an overview of QA and testing...
Ensuring Product Excellence: How Windchill PLM Streamlines Quality, Risk, and Compliance Management
When working in product development, balancing innovation with quality, risk management, and compliance is critical. Companies must meet stringent regulatory requirements, address increasing product complexity, and ensure their processes accelerate innovation. PTC’s...
Beyond Timelines and Budgets: How Strategic Project Management Accelerates Market Readiness
Meeting deadlines and staying within a budget are important to business success, but continuous achievement requires more than these two factors. Modern organizations understand that a strategic project manager (PM) is vital to project success. A PM’s role extends...
Using Creo to Meet Stringent Regulatory Requirements in Medical Device Engineering
All medical device manufacturers understand that precision, safety, and regulatory compliance are vital in their industry. Without these precautions, individuals could be harmed. These strict requirements and extended life cycles often make the time between designing...
Blog: Leveraging PTC’s Integrity Platform for IEC 62304 Compliance
SPK and Associates leverage PTC’s Integrity platform to help Medical Device companies develop software efficiently while achieving IEC 62304 compliance.