A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development must be integrated with a Quality Management System.
Let’s look at a typical business scenario:
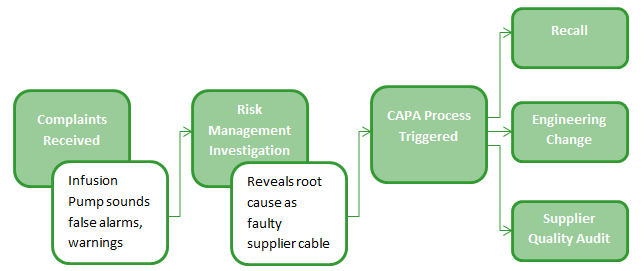
Consider how many different workflow processes and were touched in this one example. How much time was spent searching for information in different systems? How many people were required to key in information manually into multiple tools?
Siloed processes implemented with point solutions inevitably fail to scale. No single point solution suite can meet 21-CFR-820 requirements completely as they ignore critical interactions between product design, quality and manufacturing:
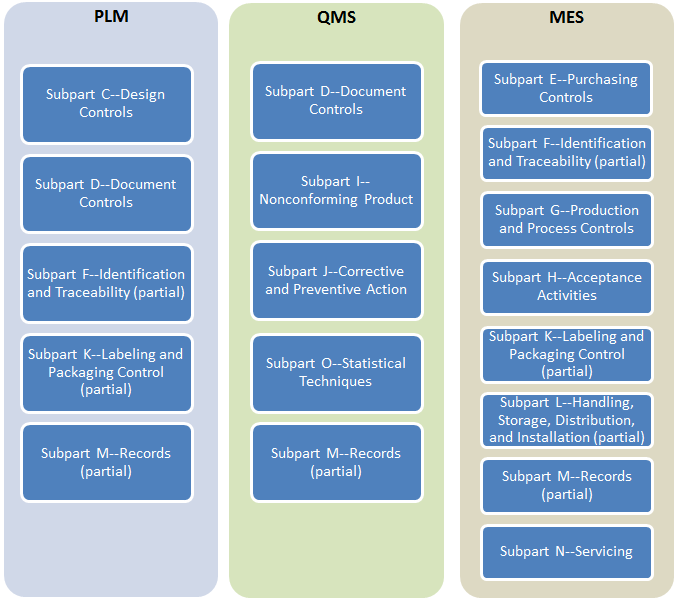
As disparate data stores for CAD files, DHFs, DMRs, CAPAs, NCRs, to name a few, grow rapidly, they become increasingly difficult to migrate and integrate.
This increases the risk of adverse business impacts like delayed product launches, product recalls, FDA 483s and warning letters and we hear:
- “Engineers are spending more time on paper work than product design!”
- “Pulling documents for an FDA audit or a 510k submission is a nightmare!”
- “We are spending more and more on IT tools but we still can’t find the right information!”
These issues can be mitigated by adopting a comprehensive Product Lifecycle Management (PLM) solution that spans the entire product development process as well as most of the quality systems processes. Additionally, end-to-end PLM solutions reflect industry best practices thus helping to increase the organization’s process maturity.
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.






