Many customers of SPK & Associates utilize the IBM/Rational suite of products. As IBM partners we are often asked to define, configure and deploy IBM tools and processes.
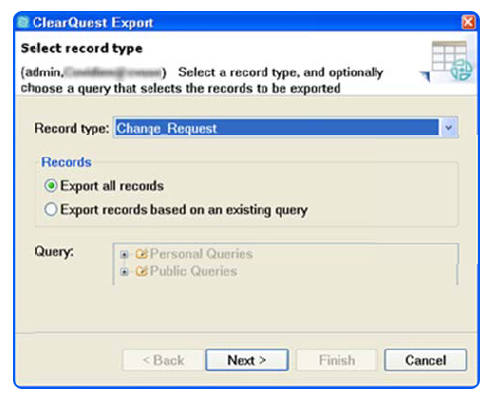
SPK has provided Engineering Technology Services to Medical Device companies for over 12 years. Recently one of our medical device accounts asked us to perform a migration of one of their legacy ClearQuest user databases into a new system. ClearQuest is IBM/Rational’s popular bug tracking and workflow management solution within their Application Lifecycle Management (ALM) suite.
Fortunately we were very familiar with the new system as we had just completed the design and deployment of its new schema and work flow. Our engineering experts were able to perform the task very quickly, and do it over a weekend to minimize disruption.
If you’re faced with a similar challenge, we invite you to download our step by step paper, so you too can migrate your ClearQuest user database in 7 steps.
Subscribe to our blog to keep informed on IBM ClearQuest, defect tracking, and other topics of interest related to Software Engineering.