As technology has advanced, it’s become more complex — and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal.
However, in industries such as the medical device or the aeronautic industry, collating and managing the data produced from the different stages and processes involved in developing a product is essential. Since these industries build products which need to be safe, robust and reliable, it is mandatory for compliance purposes that all product-related knowledge and information is collated, processed and managed.
This need to generate and maintain large amounts of product-related data can be burdensome on the development process, however. If a manual or ad-hoc process for generating compliance data is used, then innovation will be stifled or key data will be lost as executing the process of data gathering becomes difficult and ultimately ignored.
The success of any process is directly linked to the ease of its use. Once a process becomes an obstacle to productivity, mistakes (intentional or unintentional) will be made as engineers strive to save time.
The solution to enabling engineers to design, develop and innovate and yet store and manage the knowledge, processes and data needed for regulatory compliance is to implement a Product Lifecycle Management strategy.
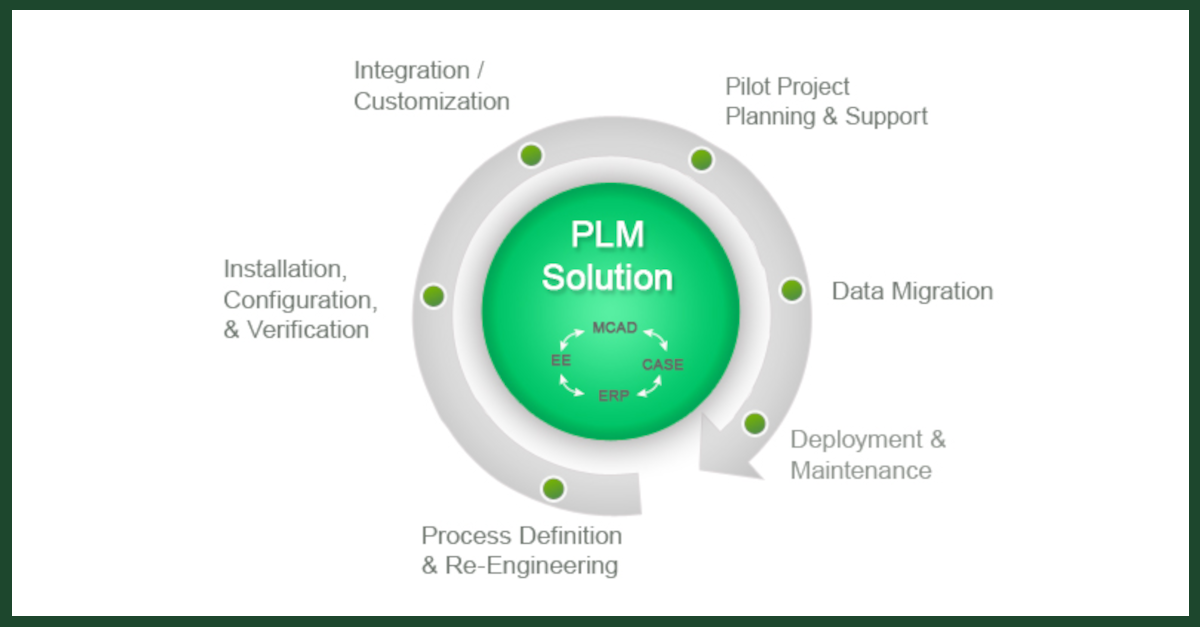
PLM is an information driven strategy which allows organizations to create, develop, deliver, support and retire products. By using integrated tools and a central repository for all product related information, this process captures all the knowledge, decisions and information generated throughout a product’s entire lifecycle.
Because it is integrated and provides tools for use at every stage of the product’s lifecycle, this approach removes the burden of compliance data gathering and ensures that all the key information is captured from the different and separate stages of the products creation, from conception to retirement.
As a development model, PLM integrates the people, the data, the processes, and the business into a product-related information backbone. From this information; store management, design and engineering decisions can be made based on what has happened previously in the product lifecycle but also based on the knowledge acquired in the creation of other products in the business. Simultaneously, compliance data is available for submission to the appropriate regulatory bodies.
Implementing PLM in your business brings many advantages, however it needs to be implemented correctly to reap all of the benefits. The best way to do this is to partner with experts who understand PLM and utilize different and cutting-edge PLM tools available like Dassault’s ENOVIA solution.
Next Steps:
- Contact SPK and Associates to see how we can help your organization with our ALM, PLM, and Engineering Tools Support services.
- Read our White Papers & Case Studies for examples of how SPK leverages technology to advance engineering and business for our clients.